What Is Keratoconus?
Keratoconus, often referred to as “KC,” is a non-inflammatory eye condition in which the typically round dome-shaped cornea progressively thins and weakens, causing the development of a cone-like bulge and optical irregularity of the cornea. This causes “static” in your vision and can result in significant visual impairment.
Symptoms
Keratoconus typically first appears in individuals who are in their late teens or early twenties, and may progress for 10-20 years and then slow or stabilize. Each eye may be affected differently. In the early stages of keratoconus, people might experience:
• Slight blurring of vision
• Distortion of vision
• Increased sensitivity to light
The cornea is responsible for focusing most of the light that comes into the eye. Therefore, abnormalities of the cornea, such as keratoconus, can have a major impact on how an individual sees the world, making simple tasks such as driving a car or reading a book very difficult.1
Keratoconus:
• Can result in significant vision loss
• May lead to corneal transplant in severe cases
• Affects both males and females
• Affects all ethnicities
• 10% of people with KC have affected relatives
• People with Down syndrome are 20 times more likely to be affected
You can find more information from the National Keratoconus Foundation at www.NKCF.org.
Treatment
What Is iLink™ Corneal Cross-Linking?
Lehmann Eye Center offers iLink™ corneal cross-linking - a minimally invasive outpatient procedure that combines the use of ultraviolet light and specially formulated eye drops to stiffen and strengthen corneas that have been weakened by disease or refractive surgery. Cross-linking is considered the standard of care around the world for progressive keratoconus and corneal ectasia following refractive surgery.2
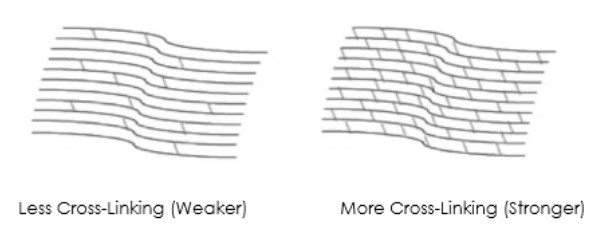
Corneal Cross-Linking3
• Creates new corneal collagen cross-links
• Results in a shortening and thickening of the collagen fibrils
• Leads to the stiffening of the cornea
Riboflavin
Under the conditions used for iLink™ corneal cross-linking, specially formulated pharmaceutical-strength riboflavin eye drops called Photrexa® (riboflavin 5’-phosphate ophthalmic solution) and Photrexa® Viscous (riboflavin 5’-phosphate in 20% dextran ophthalmic solution) help enable the cross-linking reaction.
Ultraviolet Light (UV)
iLink™ corneal cross-linking applies an artificial source of ultraviolet light from a machine called the KXL System once the cornea has been soaked in the Photrexa® and Photrexa® Viscous eye drops. This process works to stiffen the cornea by increasing the number of molecular bonds, or cross-links, in the collagen.
Combining Riboflavin and UV Light
Using Photrexa® and Photrexa® Viscous riboflavin eye drops, combined with ultraviolet light from the KXL system, the iLink™ procedure stiffens and strengthens the cornea to slow or halt progressive keratoconus.
Is Cross-Linking Right for Me?
Patients who have been diagnosed with progressive keratoconus or corneal ectasia following refractive surgery should ask their doctor about iLink™ corneal cross-linking.
Lehmann Eye Center is proud to offer patients the first and only FDA-approved therapeutic solution for the treatment of progressive keratoconus. Now, patients who once had little to no therapeutic option to treat keratoconus have the opportunity to slow or halt the progression of this sight-threatening disease.
Is iLink™ Covered by Insurance?
The medical necessity of iLink™ has become widely recognized. As a result, commercial insurance coverage for the procedure is now over 95% in the United States.
For more information about the iLink™ procedure for the treatment of keratoconus and corneal ectasia following refractive surgery, call our office or visit livingwithkeratoconus.com.
References:
1. National Keratoconus Foundation.
2. Gomes JAP, Tan D, Rapuano CJ, et al. Global consensus on keratoconus and ectatic diseases. Cornea. 2015;34(4):359-369.
3. Beshwati IM, O’Donnell C, Radhakrishnan H. Biomechanical properties of corneal tissue after ultraviolet A-riboflavin crosslinking. J Cataract Refract Surg. 2013;39(3):451-462.